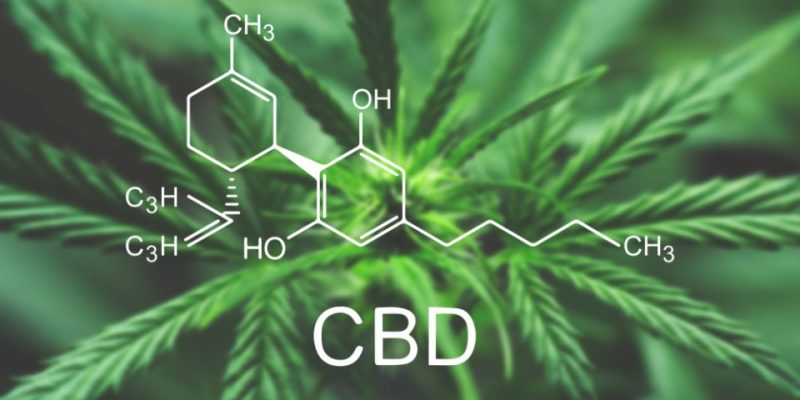
DeFloria said Monday that the Federal Drug Administration has stamped the firm’s application to begin a Phase 2 clinical trial for AJA001, an oral cannabinoid drug being made to treat symptoms of autism spectrum disorder.
The company, which was formed in 2023 as a joint-venture among Charlotte’s Web (OTCQX: CWBHF), Ajna…
Please login to read all 373 words.